[ad_1]
By Boro Dropulić, Ph.D., MBA, and Rimas Orentas, Ph.D.

Development of chimeric antigen receptor T cells — more commonly known as CAR T cell therapy — has emerged as a driving force in immunotherapy with far-reaching implications in oncology and fighting infections. The National Cancer Institute says this mode of treatment has “generated substantial excitement among researchers and oncologists.”1 The notion of “supercharging” the body’s immune system offers an effective treatment that leads to fewer side effects.
CAR T cell therapy entails splicing antibodies into T cells so they can kill target cancer cells, as an example. Approved treatments so far in this growing pipeline include Kymriah (tisagenlecleucel), Yescarta (axicabtagene ciloceucel), Tecartus (brexucabtagene autoleucel), and Breyanzi (lisocabtagene maraleucel). Many of these therapies target hematologic cancers, such as different forms of leukemia or Hodgkin’s disease.
High costs are endemic to these treatments, leading to a large gap in healthcare between those who can afford to pay and those who cannot, particularly in underserved communities. Personalized medicine shows how traditional economies of scale are not easily applied in this field. The process of manufacturing CAR T cell therapies is complex, as there needs to be a chain of custody of a patient’s T cells during the process of collecting blood and reinjecting the treated T cells back to the patient.
The Current Manufacturing Process
Currently, CAR-T manufacturing is dependent on complex, centralized processes. Like most gene therapies, most CAR T cell manufacturing for clinical trials is conducted at major academic medical centers. The multistep process starts with collecting the patient’s blood via apheresis, allowing T cells to be collected. Patients need white blood cells to be extracted for collection and processed by trained personnel in a sterile environment, where they are cultured with a gene vector and stimulated with antibodies to activate them. Using automated systems, T cells are enriched to eliminate cells that inhibit expansion or circulating tumor cells. T cells are activated by using activation reagents, which are antibodies complexed to nanoparticles. After activation, the cells are engineered to express the selected CAR gene(s) generally using viral vectors, such as lentiviral vectors. Finally, CAR T cell expansion is done in a chamber with a cell culture medium until the final dose is reached.
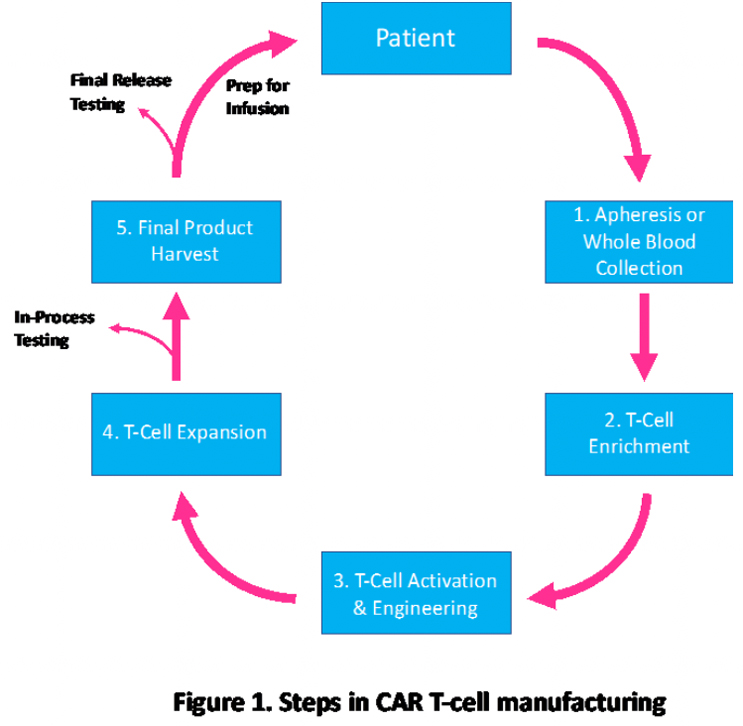
The fragmented workflow model of developing CAR-T therapies causes problems that are further complicated by shipping and handling of the T cells.
The Benefits Of Decentralizing Our Manufacturing Processes
However, changes in manufacturing processes — namely decentralizing them — can reduce costs and complexity. As Dr. Shannon L. Maude said in a Q&A with Clinical Advances in Hematology & Oncology, “It was important to learn that it is possible to export CAR T cell therapy to more patients and more broadly.”2 Decentralized manufacturing also reduces the “vein-to-vein time” that can improve clinical outcomes, as studies show the importance of using “fresh product.” Also, centralized manufacturing of CAR-T revealed some weaknesses in getting the treatment to patients, despite representing advances in clinical outcomes for patients receiving treatment.3,4
Many hospitals are enhancing their capabilities to develop CAR T cell treatments with new equipment, effectively eliminating several steps of treatment with the help of automation. The cost of developing CAR-T therapies can be whittled down to the process and equipment. While that is not cheap, doing so can remove significant costs from the $350,000 to $500,000 tied to this therapy. Figure 2 shows how decentralized CAR-T unit costs can be lowered as more patients use the facility.5 The International Journal of Cancer published an analysis in June 2020 that found: “Decentralized T cell production might be a less costly and more efficient alternative to the current centralized production mode that requires a high acquisition cost.”
Figure 2
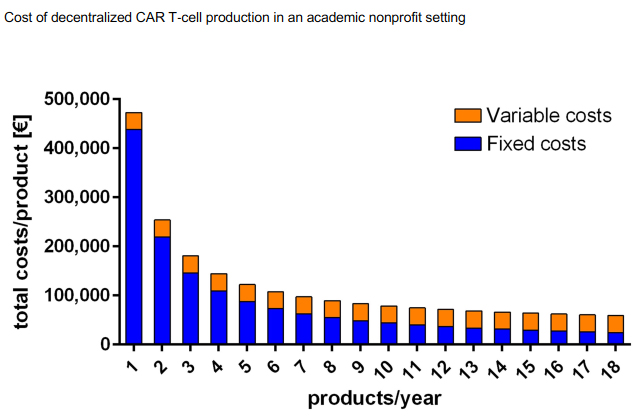
Figure used with permission from the International Journal of Cancer.5
CAR-T manufacturing can be brought closer to the patient to offset some of the complexities and difficulties of the manufacturing process, leading to quicker care. This becomes a significant treatment factor for patients dealing with rapidly progressing diseases. Also, some data have shown that patients respond better to “fresh” treatments.
In a Phase 1 study published in Blood in November 2021,6 decreasing the time from apheresis to infusion can help patients that have rapidly progressing B cell lymphoma. The study on 28 patients, who did not benefit from two or more previous lines of therapy, were given AntiCD19 CAR T cells with a mean cell infusion time of 13 days. The study found that 81% presented a disease response and 71% had achieved a complete response. Among the patients achieving a complete response, 94% had sustained remission at 12 months. Regarding side effects, more than half the patients (55%) had experienced cytokine release syndrome – a fairly common side effect from CAR-T therapy when used for oncological indications. “On-site manufacture was successful in all (patients). This strategy, in combination with fresh product infusion, can make CAR T cell therapy rapidly available for (patients) with high-risk recurring/refractory B cell lymphoma,” the study showed.6 The simplified logistics also provide flexibility to make decisions based upon the patient’s disease status, such as splitting dosages.
The approach of one-batch, one-patient manufacturing creates challenges to scaling cell therapies in a commercial setting. The advantages, however, become apparent, as long as cGMP is met. When manufacturing on-site, a few products can be made simultaneously, significantly reducing the need for multiple layers of oversight to ensure a correct chain of custody. Local manufacturing gives the ability to deliver fresh CAR T cells to patients, which can affect the “quality” of the product. Fully automated, closed CAR T cell manufacturing platforms should be utilized to boost the level of freshness of the cells. Automated platforms can significantly reduce labor and renovation costs and minimize the risk of microbial contamination and provide manufacturing consistency.
In-house product testing must be done to ensure fresh treatments get to patients in a timely manner. Testing can include flow cytometry, sterility assays and functional assays, or cell cytotoxicity. Depending on how much testing is done in-house, a qPCR system may be required. For patients unable to receive fresh CAR T cells, cryomedia should be used.
Regulatory Considerations
A place-of-care regulatory framework would balance requirements for the control of these products to ensure levels of safety equivalent to current products, which are manufactured using the centralized paradigm. This framework would avoid unnecessary regulatory barriers and enable broader access to CAR-T for patients. Providing a place-of-care regulatory framework for decentralized manufacturing provides broader patient access while ensuring safety levels equivalent to current products. This type of creative regulation enables a broader rollout of decentralized manufacturing.
In terms of regulatory compliance, manufacturers need to follow all cGMP regulations for CAR T cells. This includes an IND to be approved by the FDA when working with human subjects. All details of the cell manufacturing, components, determination methods, and product labeling information are to be included in the chemistry, manufacturing, and control section of the IND. For non-GMP grade reagents, Certificates of Analysis (COA) need to be provided in the IND application.
In March 2022, the FDA published draft guidance for manufacturing CAR T cell products, suggesting that multisite manufacturing of these products needs to show that analytical methods are comparable across different sites and that any IND describes any differences in the manufacturing process across different sites.7 The FDA recommends that each of the testing sites use the same standard operating procedures, reagents, and equipment and that standard materials should be used to calibrate equipment.
European authorities are conceiving a hospital exemption to allow clinical centers to develop and market their own therapies. El Hospital Clinic de Barcelona received approval for commercializing a CD19 CAR-T therapy (ARI-0001) for patients aged 25 up with lymphoblastic leukemia. Additionally, the U.K.’s Medicines and Healthcare Products Regulatory Agency is seeking a regulatory framework for point of care decentralized manufacturing. With respect to quality control testing of the final product, the U.K. officials acknowledge that in a decentralized manufacturing approach, “there is likely to be no time at the end of manufacture for Quality Control testing and Qualified Person certification prior to supply.”
Quality Considerations
One of the aims of cGMP rules is to ensure consistent quality of the products being produced with deep reviews of all aspects of manufacturing. To assist with development, a lab quality program should be in place to ensure consistent manufacturing and help identify alternative sources should a shortage occur. Staff must be trained around aseptic culture, tissue culture, cell counting, cryopreservation and thawing. If the facility has its own flow cytometry, added training should be necessary. Finally, all training records need to be documented.
Three assays must be performed to allow CAR-T treatments to be used. These assays include a test for sterility, a test for cell composition, and a test that includes two PCR assays, providing information on dosage and safety of the recombinant viral vector. Qualified staff should review COAs and must validate all assays to be used for patient administrated to determine whether the product can be administered. After all aspects of the CAR T cells have been checked, the product is good to be released.
Decentralized manufacturing can lead to consistent quality of certain processes. Nature Communications published a 2021 study that found that place-of-care manufacturing produces consistent CAR T cells at multiple sites for patients with B cell malignancies.8 The study found that place-of-care manufacturing showed successful production rates of 96% at one site and 100% at another. The decentralized approach to manufacturing increases the likelihood of receiving CAR therapy, and patients who would not be eligible due to disease progression while waiting for (centralized) product manufacture may also be more likely to suffer toxicity or infections.
Conclusion
We are in a revolutionary time in medicine as the seeds from mapping the human genome approximately 20 years ago are starting to flourish. What seemed unimaginable in treating disease has now evolved into a series of approved products that target conditions that had few care options a generation ago. CAR-T represents significant promise in patient care, and we are in the early stages of uncovering its potential.
We must continue to press on so the technological innovations and the decrease in cost to manufacture CAR-Ts will lead to expanded access to this mode of treatment for all patients who need these life-saving therapies. High costs are a tremendous barrier to care, but these costs can be reduced without compromising the quality of care and medical outcomes. Access to training and equipment can essentially increase the supply of hospitals and healthcare providers providing the therapy beyond the biggest medical centers.
CAR-T has provided us with many tools to fight different types of leukemia and lymphoma. We are in the very early stages of its capabilities as researchers examine how it can be deployed to target solid tumors or infectious diseases. Expanding the settings that can use CAR-T can allow this therapeutic approach to reach its fullest potential. A regulatory pathway must be established to help encourage decentralized production of CAR-T therapies to help not only manage costs but improve patient care, especially as CAR-T’s capabilities become more pronounced in transforming immunotherapy.
References
- National Cancer Institute, “CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers,” https://www.cancer.gov/about-cancer/treatment/research/car-t-cells (accessed Aug. 8, 2022).
- Maude, Shannon L., “Tisagenlecleucel in Pediatric Patients with Acute Lymphoblastic Leukemia,” Clinical Advances in Hematology & Oncology, Oct. 2018, Vol. 16, Issue 10.
https://www.hematologyandoncology.net/archives/october-2018/tisagenlecleucel-in-pediatric-patients-with-acute-lymphoblastic-leukemia/ (accessed Aug. 23). - Schuster, Stephen, Jakub Svoboda, et al. “Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas,” New England Journal of Medicine, Dec. 28, 2017. https://www.nejm.org/doi/full/10.1056/NEJMoa1708566 (accessed Aug. 23, 2022).
- Majzner, R. G. & Mackall, C. L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25, 1341–1355 (2019).
- Ran, Tao and Stefan B. Eichmuller et al. “Cost of decentralized CAR T-Cell production in an academic nonprofit setting,” International Journal of Cancer, June 14, 2020, https://doi.org/10.1002/ijc.33156 (accessed Aug. 9, 2022).
- Caimi, Paolo F., Armin Ghobadi, et al. “Final Results of a Phase 1 Study of AntiCD19 CAR-T Cels with FNFRSF19 Transmembrane Domain,” Blood, Nov. 5,2021 https://doi.org/10.1182/blood-2021-151155 (accessed Aug. 8, 2022).
- U.S. Food and Drug Administration. “Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products.” Draft guidance issued March 2022. https://www.fda.gov/media/156896/download (accessed Aug. 23, 2022).
- Maschan, Michael, Paolo F. Caimi, et al. “Multiple site place-of-care manufactured anti-CD19 CAR-T cells induce high remission rates in B-cell malignancy patients,” Nature Communications, Dec. 10, 2021, https://www.nature.com/articles/s41467-021-27312-6 (accessed Aug. 23, 2022).
About The Authors:
 Boro Dropulić, Ph.D., MBA, is co-founder and executive director of Caring Cross. Previously, he founded ViRxSys and Lentigen, and led the development of a global place-of-care network of clinical centers that were able to manufacture CAR-T cell products and demonstrate their therapeutic benefits in clinical trials. He received his Ph.D. from the University of Western Australia and his MBA from the Johns Hopkins University. He has been in the gene therapy field since the late 1980s.
Boro Dropulić, Ph.D., MBA, is co-founder and executive director of Caring Cross. Previously, he founded ViRxSys and Lentigen, and led the development of a global place-of-care network of clinical centers that were able to manufacture CAR-T cell products and demonstrate their therapeutic benefits in clinical trials. He received his Ph.D. from the University of Western Australia and his MBA from the Johns Hopkins University. He has been in the gene therapy field since the late 1980s.
 Rimas Orentas, Ph.D., is an investigator at the Ben Towne Center for Childhood Cancer Research, Seattle Children’s Research Institute; professor of pediatrics and laboratory medicine and pathology at the University of Washington School of Medicine; and chair of the Board of Directors for Caring Cross. He received his Ph.D. from The Johns Hopkins University School of Medicine. Previously, he worked in academia, at the Pediatric Oncology Branch of the NCI, and at Lentigen Corporation.
Rimas Orentas, Ph.D., is an investigator at the Ben Towne Center for Childhood Cancer Research, Seattle Children’s Research Institute; professor of pediatrics and laboratory medicine and pathology at the University of Washington School of Medicine; and chair of the Board of Directors for Caring Cross. He received his Ph.D. from The Johns Hopkins University School of Medicine. Previously, he worked in academia, at the Pediatric Oncology Branch of the NCI, and at Lentigen Corporation.
[ad_2]
Source link








