[ad_1]
Participants and evaluated variables
The enrolled participants were recipients of the first and second doses of mRNA COVID-19 vaccines, mRNA-1273 (Moderna Corp, Cambridge, USA)12, who were vaccinated at a single large-scale mass-vaccination center located in Sendai City between August and November 2021. All the vaccine recipients were aged ≥ 16 years. Data on age and sex were collected from all recipients. Additional information regarding the details of the adverse events was collected from those who had acute adverse events at the vaccination center. The acute adverse events were categorized into the following diagnoses at the site by the doctors who examined the patients: vasovagal syncope/presyncope as a representative manifestation of immunization stress-related response, acute allergic reaction, anaphylaxis, and other conditions. The study design is illustrated in Fig. 1.
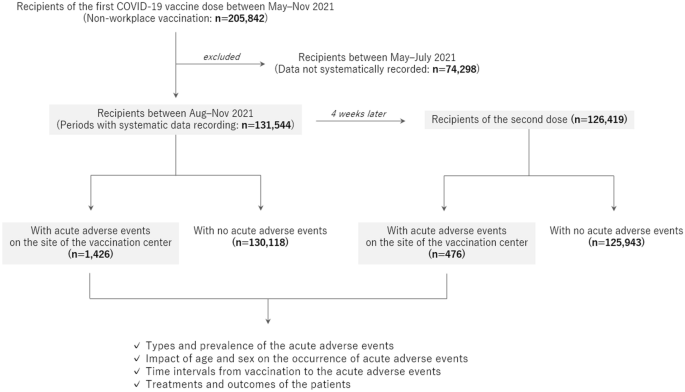
Flow diagram of the study design. All recipients of the Moderna mRNA vaccine against COVID-19 at a single mass vaccination center were enrolled in the study. Detailed information on the occurrence of acute adverse events at the site of the center was evaluated after stratification by age group, sex, and the time of vaccination.
For reference, individuals with a previous medical history of vasovagal syncope/presyncope, including those with that after the first COVID-19 vaccine dose, were recommended to receive the vaccine dose in the supine position, laying on the bed in the first-aid office of the testing center. Therefore, the recipients who had vasovagal syncope/presyncope after the first vaccine dose received the second vaccine dose in the supine position. Information on the type of body position used during the vaccine injection (supine or normal sitting position) was collected.
Observation time on site after injection
All of the vaccine recipients were asked to remain in the follow-up booth of the vaccination center for at least 15 min after the injection, and those individuals who had past medical histories of severe food or drug allergic reactions, including anaphylaxis, were asked to stay in the follow-up booth for at least 30 min after the injection. Individuals who were anxious about the development of acute adverse events were also advised to stay at the follow-up booth for at least 30 min. After the first vaccine dose, all recipients were then guided to another booth to be explained the next vaccine dose schedule and to make a reservation for the second vaccine dose. Therefore, almost all vaccine recipients after the first vaccine dose stayed at the vaccination center for at least 30 min. Meanwhile, many recipients of the second vaccine dose could have left the center before 30 min after the injection.
Statistical analysis
Qualitative variables were compared between the two groups using the chi-square test or Fisher’s exact test, according to the sample size of each cell. The 95% confidence interval (CI) was further calculated for the prevalence of each symptom among all vaccine recipients. As multiple comparisons were performed simultaneously, p < 0.001 was considered statistically significant in this study. Statistical analyses were performed using the R statistical software (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria).
Ethical standards statement
This study was approved by the institutional review board of the Tohoku University Graduate School of Medicine (approval number: 2021-1-566). All processes of the present study were performed in accordance with the ethical standards of the Declaration of Helsinki of 1964 and its later amendments. Informed consent was obtained from all of the participants.
[ad_2]
Source link