[ad_1]
At first glance, some research articles published in the journal may appear to lack substantial novelty.
In the book The Structure of Scientific Revolutions, Thomas Kuhn emphasized that “novelty emerges only with difficulty, manifested by resistance, against a background provided by expectation”1. Indeed, discovering new knowledge or making something new is laborious. And many scientists have seen their novel scientific output being met with some degree of opposition, especially when what’s new challenges established understanding or methodology, or puts at risk someone’s scientific agenda, perceived standing in the community or financial interests.
In practice, Kuhn’s framing of the ‘background of expectations’ can be portrayed as the battlefield where authors, reviewers and editors meet when a paper is assessed for publication. What is actually new in the work? Is the novelty of the findings, or the innovation reported, sufficient for the perceived status of the journal? Is the advance impactful? For some papers, the answers to these questions do not lead to meaningful debate: most published research is incremental — as it should be; after all, knowledge and scientific developments typically evolve a small step at a time, and academic incentives favour those who publish more. Yet, for the minority of papers that push the boundaries of science and technology harder and further, the assessments of novelty and value can differ substantially. At Nature Biomedical Engineering, this is our bread and butter2. Yet not because most of the papers that we publish are met with resistance as to the claimed degree of novelty; rather, most often, the advance or impact may not be apparent3. Why? There are, at least, three factors.
First, multidisciplinary and interdisciplinary work (and most work published in interdisciplinary journals4,5) is harder to assess for most editors, reviewers and readers. Second, applied work — as is the case for most papers published in technology-centred journals such as Nature Biomedical Engineering — largely aims at problem-solving rather than at knowledge discovery. Hence, the main source of novelty may be an alternative solution to the same problem, or one that is more efficient or more robust, easier, faster or cheaper. Sometimes, the advance is to show that an expected outcome can actually be achieved, that a technology can work in humans or that a product can be scaled up. For such studies, disagreements about how novelty is perceived can, more often than not, lead to unproductive discussion or outcomes.
The second factor hints at a closely related third factor: utility. The usefulness of the outcomes of a project — a method, a device, code or a new dataset, for example — may take some time to be appreciated, especially when the target users aren’t those working in the same topic, disease area or technology. Moreover, novelty and innovation may not even be a relevant consideration in such cases. In fact, Nature Biomedical Engineering has published articles whose main contribution is to report a particularly useful benchmarking effort6, a desired resource of clinical data7, or the optimization or clinical validation of a biologic, device or algorithm.
In this issue of the journal, we have compiled four research articles reporting advances in cancer immunotherapy, and one article in immunotherapy for chronic allergic and inflammatory diseases. At first glance, for some of these papers, some readers may wonder what’s really new in this work. In the remainder of this Editorial, we offer our view as editors of the journal.
Polymeric nanoscale micelles have long been used to deliver drugs intravenously. Cathryn Nagler, Jeffrey Hubbell and colleagues now show that micelle-forming block copolymers can effectively deliver the short-chain fatty acid butyrate orally to different regions of the gastrointestinal tract to restore intestinal homeostasis in mouse models of peanut allergy and colitis. Because butyrate-producing commensal bacteria are known to protect from food allergies, and butyrate has been given to such model animals through drinking water, what’s the actual advance in this work? In short, a translationally relevant formulation for the treatment of food allergies and chronic inflammation in the gut. The authors formulated two types of water-suspensible micelle (varying in their electrical charge) to encapsulate a high dose of butyrate (masking its taste and smell) and to target its release into two different regions in the lower gut. In combination, the two types of micelle reduced the permeability of the intestinal barrier tissue in the mice, protected them from an anaphylactic response when given peanuts, and increased the abundance of protective bacteria (Fig. 1).
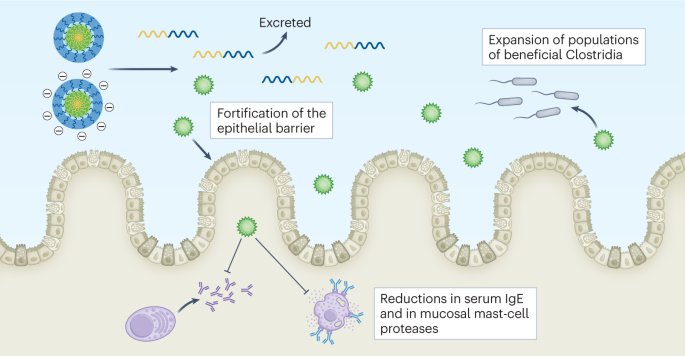
The image shows that intestinal homeostasis in mouse models of peanut allergy and colitis can be restored by using polymeric nanoscale micelles to deliver the short-chain fatty acid butyrate to the lower gastrointestinal tract. IgE, immunoglobulin E. Figure reproduced from the Article by Nagler, Hubbell and colleagues, under a Creative Commons license CC BY 4.0.
In another article, James Moon and colleagues report that intratumourally injected hollow nanoparticles displaying the microbial polysaccharide mannan downregulate the fraction of regulatory T cells in the tumour microenvironment and induce antitumour responses mediated by T helper 17 cells. The novelty of this work is predominantly mechanistic: it shows, in multiple tumour models, that T-helper-17 immune responses in tumours can be leveraged for cancer immunotherapy. However, intratumoural delivery of the treatment reduces its translational potential.
The suppression of regulatory T cells and the attraction and stimulation of effector T cells in the tumour microenvironment can also be achieved, as shown by Manish Butte, Fatemeh Majedi and colleagues, via a different strategy: an implanted biomaterial with advantageous functionality and promising outcomes. The scaffold, placed peritumourally, releases small molecules (including an inhibitor of transforming growth factor β) and antibodies. The researchers also show that, in mice bearing aggressive skin or breast tumours, the scaffolds lead to an abscopal effect on distant metastases, hindering tumour recurrence. However, the make-up of the scaffold would need to be simplified if this immunotherapy strategy (not absolutely novel, yet nascent) was to be pursued as a therapeutic product.
The two other articles in the issue provide solutions to challenging problems in cancer immunotherapy: the heterodimeric cell-surface glycoprotein CD98 (with roles in the transport of aromatic and branched-chain amino acids and in integrin signalling) is highly expressed in cancer cells, and hence is a vulnerable target protein for immunotherapies. However, CD98 is also widely expressed in most tissues, which makes higher doses of immunotherapy necessary and increases the likelihood of on-target side effects. Jianhua Sui and co-authors show that these limitations can be overcome by an anti-CD98 antibody with pH-dependent binding. The antibody, identified via phage display, led to tumour-specific antitumour activity (without disturbing the physiological function of the glycoprotein) in multiple tumour types in CD98-humanized mice. There are many examples of antibodies with pH-dependent binding, but designing an antibody targeting CD98 specifically in human tumour cells, and dissecting the downstream mechanisms that lead to innate and adaptive antitumour immunity opens a translational path for anti-CD98 antibody-based immunotherapies.
Similarly, designing cell therapies leveraging chimaeric antigen receptors (CARs) that elicit strong antitumour effects in solid cancers is a difficult translational problem, particularly when using induced pluripotent stem cells as a cell source. Shin Kaneko and colleagues now show how CAR T cells derived from human induced pluripotent stem cells can be optimized (by designing CAR constructs targeting the co-receptor CD8αβ, and by enhancing CAR and cytokine signalling) for proliferation and persistency in solid tumours, with therapeutic outcomes (as the researchers show in multiple tumour models in mice) comparable to those induced by primary CD8 CAR T cells. The study also offers practical insight into how the make-up of the transduced CAR impacts the differentiation of the stem cells into T cells.
An analysis of citations to nearly 25 million papers and 4 million patents published over the 6 decades ending in 2010 suggests that, across areas of science and technology, innovation has become markedly less disruptive over time8, and that this is unlikely to be driven by changes in the quality of the papers or in citation practices. The authors attribute this slowdown in scientific progress to academic-career incentives pushing researchers to rely on narrower slices of knowledge. Yet another factor in this apparent trend may be the increasingly applied nature of recent disruptive technologies. After all, producing billions of doses of vaccines, creating the iPhone and making a chat version of a large language model9 do not prominently feature in the literature. Indeed, they may not be really new.
[ad_2]
Source link







